Professional Website
Human Milk Oligosaccharide (HMO) composition in relation to risk of Necrotizing Enterocolitis (NEC)
Nathan Lewis, Lars Bode
Necrotising enterocolitis (NEC) is one of the most common and often fatal intestinal disorders in preterm infants. Markers to identify at-risk infants, as well as therapies to prevent and treat NEC, are limited and urgently needed. NEC incidence is signi?cantly lower in breastfed compared with formula-fed infants. Infant formula lacks human milk oligosaccharides (HMO), such as disialyllacto-N-tetraose (DSLNT), which prevents NEC in neonatal rats. However, it is unknown if DSLNT also protects human preterm infants. We conducted a multicentre clinical cohort study and recruited 200 mothers and their very low birthweight infants that were predominantly human milkfed. We analyzed HMO composition in breast milk fed to infants over the ?rst 28 days postpartum, matched each NEC case with ?ve controls and used logistic regression and generalized estimating equation to test the hypothesis that infants who develop NEC receive milk with less DSLNT than infants who do not develop NEC. Eight infants in the cohort developed NEC (Bell stage 2 or 3). DSLNT concentrations were signi?cantly lower in almost all milk samples in NEC cases compared with controls, and its abundance could identify NEC cases prior to onset. Aggregate assessment of DSLNT over multiple days enhanced the separation of NEC cases and control subjects. DSLNT content in breast milk is a potential non-invasive marker to identify infants at risk of developing NEC, and screen high-risk donor milk. In addition, DSLNT could serve as a natural template to develop novel therapeutics against this devastating disorder.
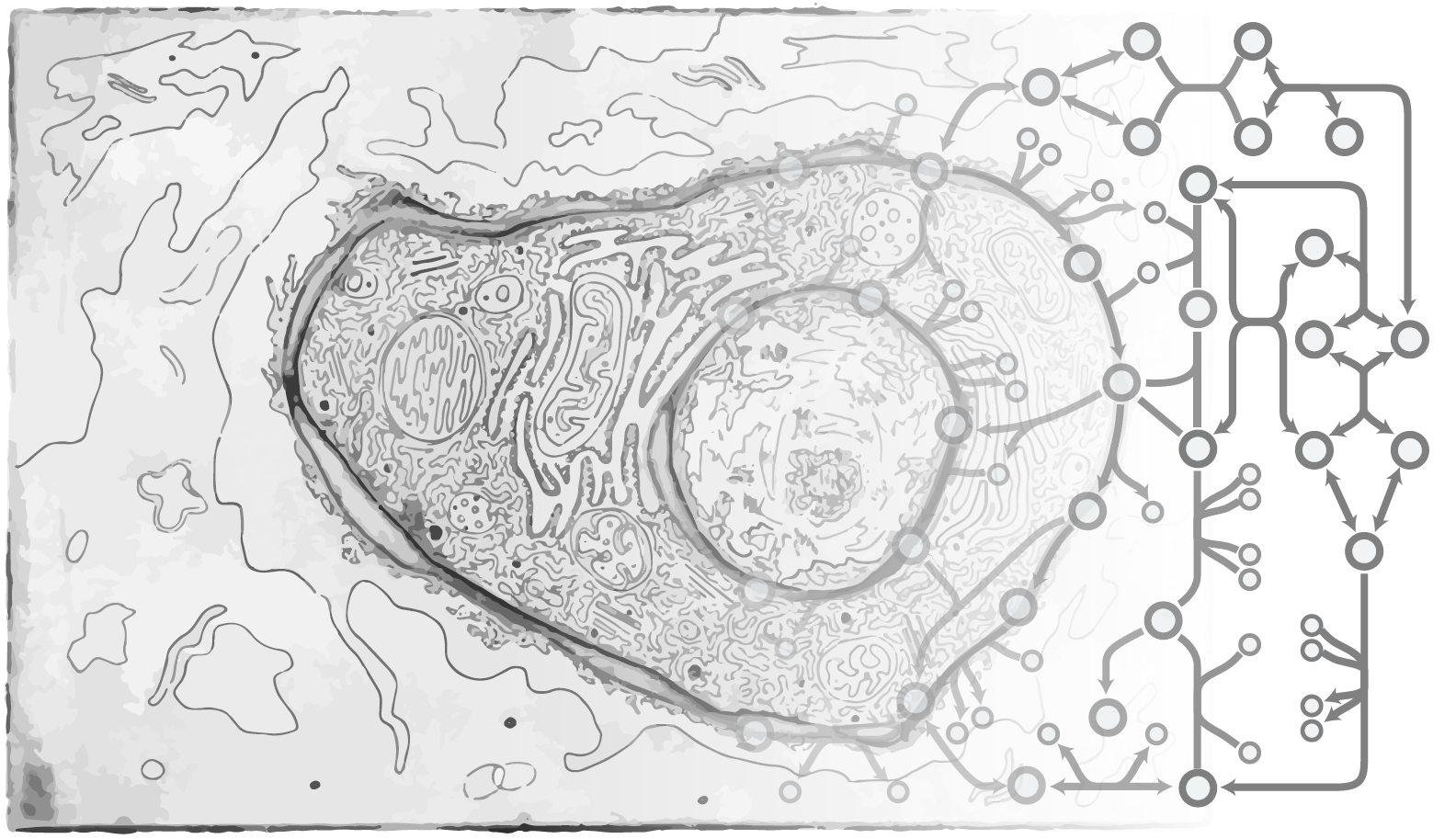
Human Milk Oligosaccharide (HMO) Biosynthesis Network Reconstruction
Nathan Lewis, Lars Bode
Human Milk Oligosaccharides (HMOs) are abundant and functionally important components of human milk. A growing body of evidence suggests these glycans have major impacts on the health and development of the breastfed infant. Although they were discovered more than half a century ago, their biosynthesis in the human mammary gland remains minimally characterized. We propose to develop a framework for predicting which glycosyltransferases are involved in HMO biosynthesis and identify candidate structures for poorly characterized HMOs. Our approach constructs and selects candidate models describing HMO biosynthesis, leveraging both metabolic and transcriptomic modeling and data. We will construct a generic metabolic network describing all feasible biosynthetic pathways of 34 potential HMO structures related to the 16 most abundant (>97% by weight) oligosaccharides found in human milk. Through the integration of HMO glycoprofiling and transcriptomics, our mechanistic modeling approach endeavors to identify the most likely HMO structures for uncharacterized HMOs, as well as specifying the association between biosynthetic reactions for those HMOs, and candidate genes for elongation and branching. These results will provide the molecular basis for HMO biosynthesis and thus can be used to guide new strategies for HMO synthesis for academic and nutritional use.