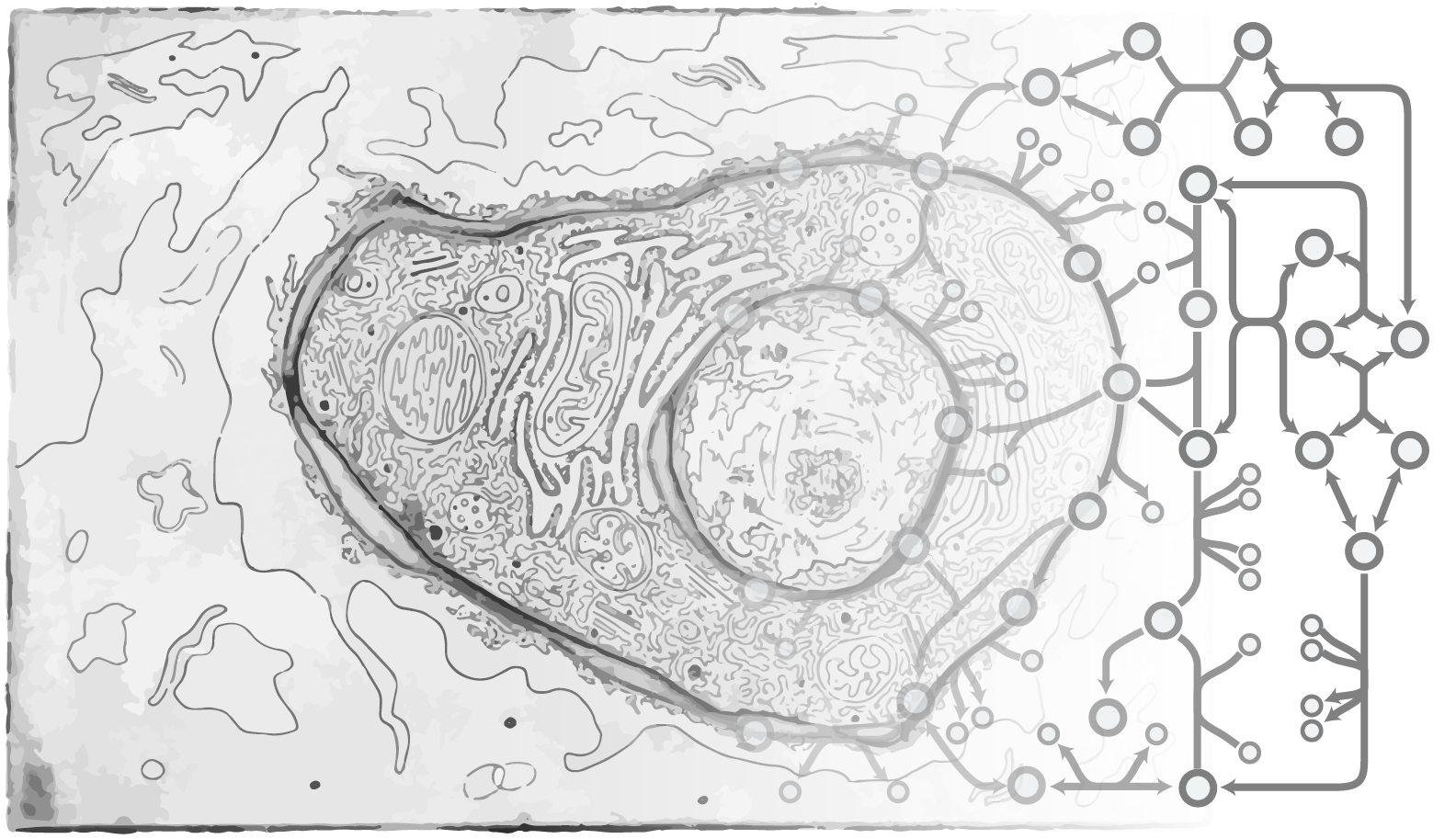
In mammalian cells, the majority of proteins are glycosylated, and most cells are coated with glycoconjugates and surrounded by complex carbohydrates in their extracellular matrix. Indeed, glycosylation is fundamentally important to most biological processes, including proper protein folding, cell attachments, pathogen infections, immune cell activities, etc. Often, the structure of these glycans is essential to the related biological functions. However, the diversity and complexity of glycosylation have made it difficult to identify how glycosylation is regulated. To address this challenge, there is a need to take a systems approach to the study of glycosylations (Spahn and Lewis, Curr Opin in Biotech, 2014). Thus, we are developing tools to unravel the complexity of glycosylation, and deploying these to understand how glycosylation is regulated.
In mammalian cells, the majority of proteins are glycosylated, and most cells are coated with glycoconjugates and surrounded by complex carbohydrates in their extracellular matrix. Indeed, glycosylation is fundamentally important to most biological processes, including proper protein folding, cell attachments, pathogen infections, immune cell activities, etc. Often, the structure of these glycans is essential to the related biological functions. However, the diversity and complexity of glycosylation have made it difficult to identify how glycosylation is regulated. To address this challenge, there is a need to take a systems approach to the study of glycosylations (Spahn and Lewis, Curr Opin in Biotech, 2014). Thus, we are developing tools to unravel the complexity of glycosylation, and deploying these to understand how glycosylation is regulated.
In these efforts we developed a computational platform using probabilistic Markov modeling of glycosylation, and demonstrate that this approach can be used to guide glycoengineering (Spahn, Met Eng, 2016; Spahn, Biotech J, 2017). It can be further used to unravel how variations in nutrients and gene regulation impact the glycans presented by a cell. We have further used our approaches to study milk oligosaccharide biosynthesis, identify new targets of proteins that regulate glycosyltransferases and glycosylation in general, and to identify health-related glycans (Autran, Gut, 2017). We augment these computational studies with the use of RNA-Seq and genome-wide CRISPR-Cas9 knock out screens to identify new regulators of glycosylation (Spahn, BioRxiv, 2017). Thus, we are introducing a systems biology perspective to glycobiology that is allowing us to unravel the complexity of this biological process and engineer the systems for therapeutical usage.